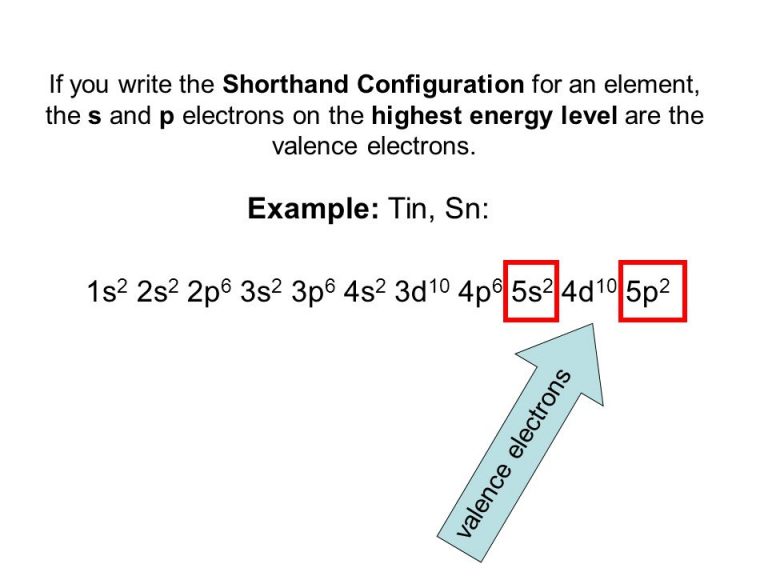
Electron Configuration Of Tin 4+ . tin electron configuration. here, the electron configuration of tin ion(sn 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2.
from periodictable.me
It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4. the electronic configuration of the tin 4+ is 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2, 3d 10, 4p 6, 4d 10, 5s 0,5p 0 as 4 electrons.tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii).
Tin Electron Configuration (Sn) with Orbital Diagram
Electron Configuration Of Tin 4+ the atomic number of tin represents the total number of electrons of tin. 60k views 3 years ago. Since the atomic number of tin is 50, the total electrons of tin are 50.tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii).
From sciencenotes.org
List of Electron Configurations of Elements Electron Configuration Of Tin 4+ tin electron configuration. Since the atomic number of tin is 50, the total electrons of tin are 50. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4. the atomic number of tin represents the. Electron Configuration Of Tin 4+.
From www.chegg.com
Solved a. Give the groundstate electron configuration of Electron Configuration Of Tin 4+ It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. here, the electron configuration of tin ion(sn 2+) is. Electron Configuration Of Tin 4+.
From www.numerade.com
SOLVED(a) What is the electron configuration for an atom of tin? (b Electron Configuration Of Tin 4+ To write the configuration for the tin (sn) and the tin ions,. tin electron configuration. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. the atomic number of tin represents the total number of electrons of tin. It shows a chemical similarity to both of its neighbors that are germanium. Electron Configuration Of Tin 4+.
From carisca.github.io
Write The Complete Groundstate Electron Configuration Of Chromium Electron Configuration Of Tin 4+ the electronic configuration of the tin 4+ is 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2, 3d 10, 4p 6, 4d 10, 5s 0,5p 0 as 4 electrons. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. 60k views 3 years ago. tin electron configuration.. Electron Configuration Of Tin 4+.
From www.numerade.com
SOLVEDGive the full electron configuration of tin. (3 pts) 6. Give the Electron Configuration Of Tin 4+ Since the atomic number of tin is 50, the total electrons of tin are 50. To write the configuration for the tin (sn) and the tin ions,. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4.. Electron Configuration Of Tin 4+.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Electron Configuration Of Tin 4+ the electronic configuration of the tin 4+ is 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2, 3d 10, 4p 6, 4d 10, 5s 0,5p 0 as 4 electrons.tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2. Electron Configuration Of Tin 4+.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Electron Configuration Of Tin 4+ It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4. the atomic number of tin represents the total number of electrons of tin. this electron configuration calculator will instantly show you the distribution of electrons. Electron Configuration Of Tin 4+.
From material-properties.org
Estroncio Protones Neutrones Electrones Configuración electrónica Electron Configuration Of Tin 4+ the atomic number of tin represents the total number of electrons of tin. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. tin electron configuration.tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. Electron Configuration Of Tin 4+.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Electron Configuration Of Tin 4+ Since the atomic number of tin is 50, the total electrons of tin are 50. tin electron configuration. here, the electron configuration of tin ion(sn 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2. the electronic configuration of the tin 4+ is 1s 2,. Electron Configuration Of Tin 4+.
From www.chegg.com
Solved 4. (a) Write an electron configuration of tin using Electron Configuration Of Tin 4+ tin electron configuration. 60k views 3 years ago. Since the atomic number of tin is 50, the total electrons of tin are 50. To write the configuration for the tin (sn) and the tin ions,. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation. Electron Configuration Of Tin 4+.
From 164427.com
Orbital Diagrams Chemistry Steps Arrange the electrons in the Electron Configuration Of Tin 4+ To write the configuration for the tin (sn) and the tin ions,. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4. this electron configuration calculator will instantly show you the distribution of electrons in the. Electron Configuration Of Tin 4+.
From www.hanlin.com
CIE A Level Chemistry复习笔记6.2.2 Oxidation States of Transition Metals翰林国际教育 Electron Configuration Of Tin 4+ tin electron configuration. the electronic configuration of the tin 4+ is 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2, 3d 10, 4p 6, 4d 10, 5s 0,5p 0 as 4 electrons. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. It shows a chemical similarity to. Electron Configuration Of Tin 4+.
From valenceelectrons.com
Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+) Electron Configuration Of Tin 4+tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii). here, the electron configuration of tin ion(sn 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s. Electron Configuration Of Tin 4+.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Electron Configuration Of Tin 4+ this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. To write the configuration for the tin (sn) and the tin ions,. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more. Electron Configuration Of Tin 4+.
From valenceelectrons.com
Electron Configuration for Selenium (Se, Se2 ion) Electron Configuration Of Tin 4+ It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4. this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of. 60k views 3 years ago. here, the electron. Electron Configuration Of Tin 4+.
From brainly.com
nitrogen has five valence electrons. consider the following electron Electron Configuration Of Tin 4+tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (ii). To write the configuration for the tin (sn) and the tin ions,. tin electron configuration. It shows a chemical similarity to both. Electron Configuration Of Tin 4+.
From general.chemistrysteps.com
Electron Configurations Chemistry Steps Electron Configuration Of Tin 4+ It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4. 60k views 3 years ago. the atomic number of tin represents the total number of electrons of tin. this electron configuration calculator will instantly. Electron Configuration Of Tin 4+.
From topblogtenz.com
How to write Electron Configuration All methods + Examples Electron Configuration Of Tin 4+ the electronic configuration of the tin 4+ is 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2, 3d 10, 4p 6, 4d 10, 5s 0,5p 0 as 4 electrons. Since the atomic number of tin is 50, the total electrons of tin are 50. To write the configuration for the tin (sn) and the tin ions,.. Electron Configuration Of Tin 4+.